This summary encapsulates a presentation given by Dr. Avigdor Abraham, MD (Pubmed publications), who serves as the Director of the Institute of Hematology and also holds the position of Co-Director of the Division of Hematology and Bone Marrow Transplantation at Sheba Medical Center. Dr. Abraham delivered this lecture at the EBMT Conference, which took place in Seville, Spain, in February 2024. (EBMT recording, minute 21:20)

At the recent EBMT conference in Seville, Dr. Avigdor Abraham, a leading hematologist from Sheba Medical Center, discussed the center's advancements in CAR-T cell therapy. The focus was on point-of-care production, specifically within a substantial cohort of patients, highlighting the challenges and achievements in treating blood cancers in Israel.
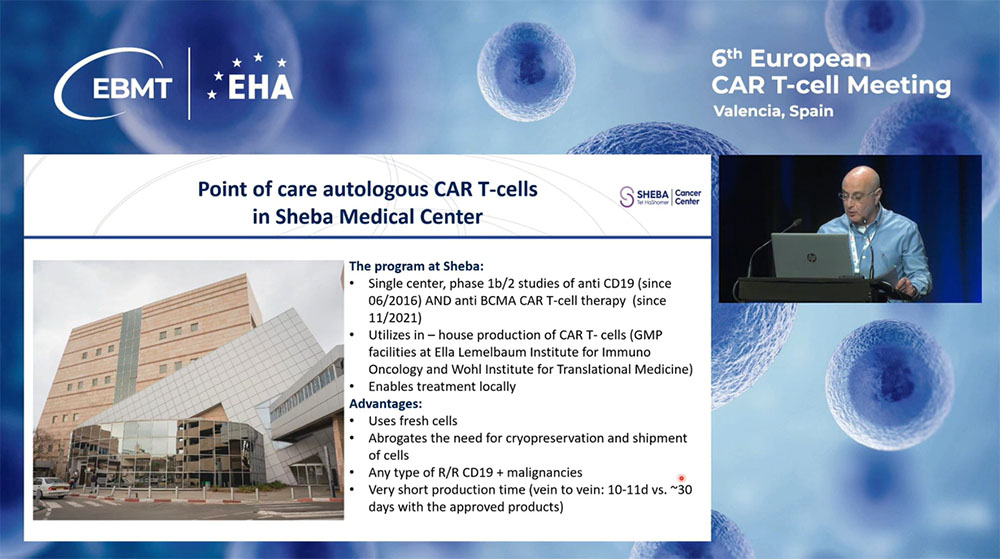
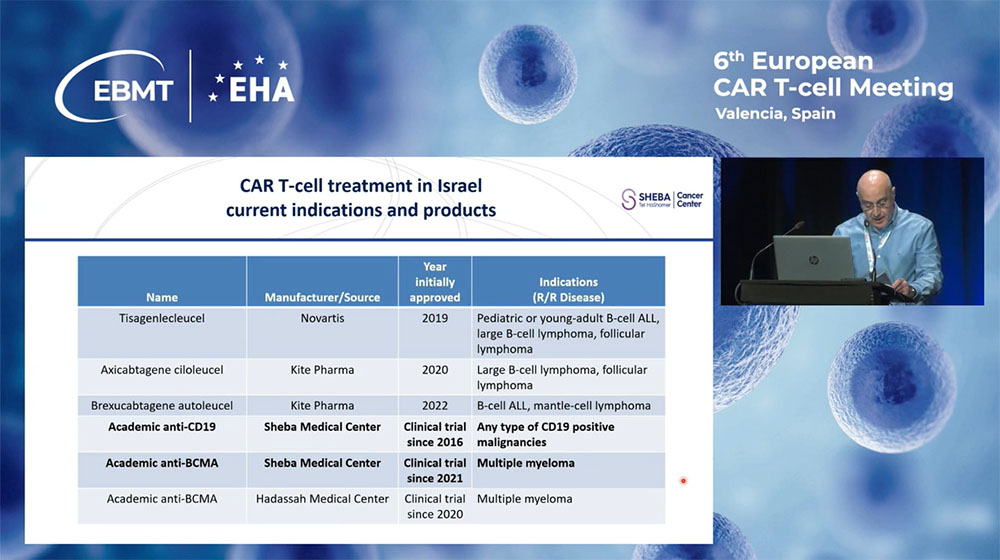
Dr. Abraham outlined the regulatory landscape, noting that while three commercial anti-CD19 CAR-T cell therapies have been approved by the Israeli Ministry of Health, the anti-BCMA CAR-T product has yet to receive approval. Additionally, Sheba Medical Center is pioneering with three academic trials producing both anti-CD19 and anti-BCMA CARs locally.
Initiated in 2016, prior to the commercial availability of CAR-T products in Israel, Sheba’s researchers have been developing anti-CD19 CAR-T cells under clinical trials targeting various hematologic malignancies. The program expanded in 2021 to include anti-BCMA CAR-T cells, emphasizing manual production in their GMP facilities. This local production method reduces costs, eliminates the need for cell shipment and preservation, and crucially shortens the "vein-to-vein" time to just 10 to 11 days.
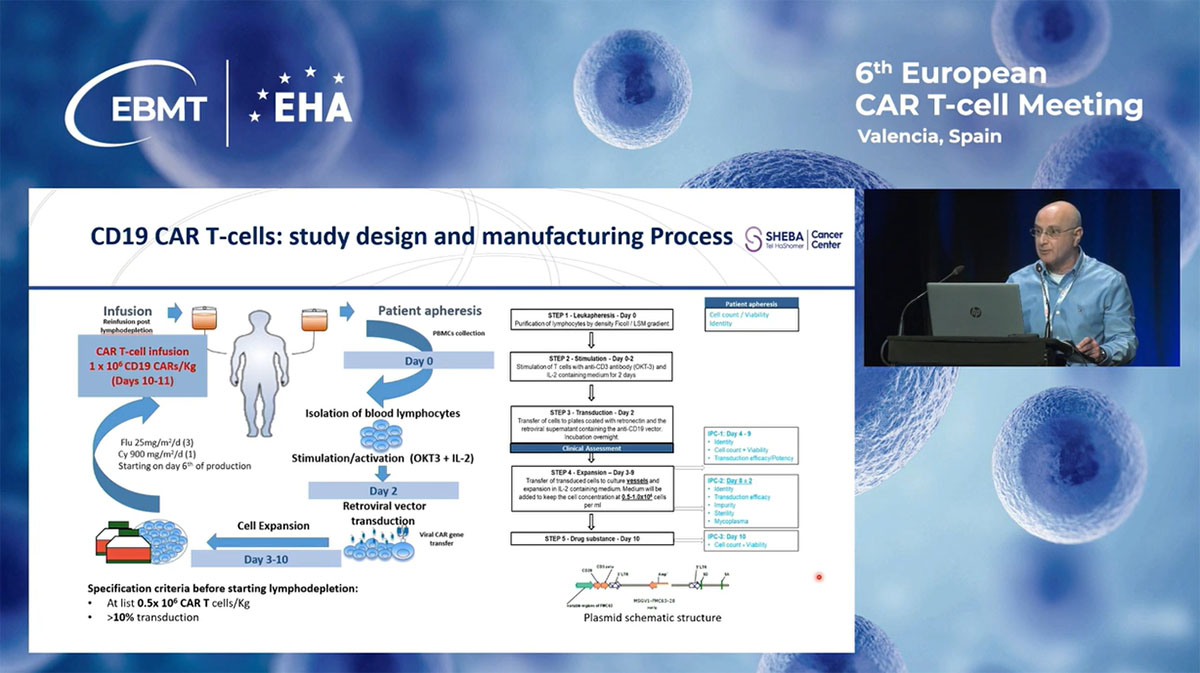
The CD19 CAR construct employed involves components like the anti-CD19 single-chain variable region, CD28 costimulatory, and CD3ζ intracellular domains. Treatment protocols involve lymphodepletion followed by the infusion of freshly transduced T-cells, targeting a rapid turnaround that enhances treatment accessibility and effectiveness.
Dr. Abraham shared significant outcomes from the center’s CAR-T therapy applications: As of January 2024, 321 patients with CD19-positive diseases were treated under this program. A subset analysis revealed a high overall response rate, particularly noting an impressive preliminary success in treating multiple myeloma with the locally produced BCMA CAR-T cells, with a complete response rate of 45% among the first 36 patients treated.
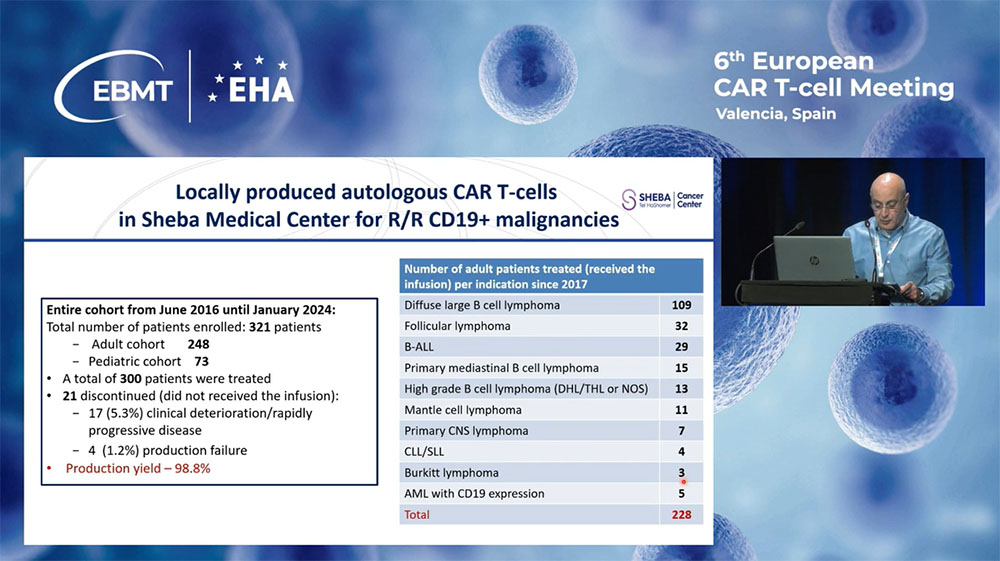
The Sheba Medical Center’s approach exemplifies how local production and innovative clinical practices can substantially improve treatment outcomes for patients with relapsed refractory blood cancers, setting a benchmark for global CAR-T cell therapy practices.











